Potsdam-Rehbrücke, 05.11.2018
New Marker Provides Insights into the Development of Type 2 Diabetes
Small chemical changes in the DNA building blocks, which may be influenceable by lifestyle factors, can reduce the amount of IGFBP2. A DIfE / DZD research team has now reported in the journal Diabetes that these epigenetic changes increase the risk of type 2 diabetes. Moreover, people with high blood levels of the binding protein IGFBP2 are less likely to develop this metabolic disorder. The changes in the blood are already detectable a few years prior to the onset of the disease.
According to the German Diabetes Health Report 2018, more than 5.7 million people in Germany suffer from type 2 diabetes. The affected individuals react inadequately to the hormone insulin, which leads to elevated blood glucose levels. This in turn can lead to strokes, heart attacks, retinal damage, kidney damage and nerve disorders. Since the metabolic disease develops gradually, initial damage has usually already occurred at the time of diagnosis. ”In the future, our findings may help to identify risk potentials for type 2 diabetes even earlier and help to counteract the disease with preventive measures," said Professor Annette Schürmann, head of the Department of Experimental Diabetology at the German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE) and speaker of the German Center for Diabetes Research (DZD).
Uncovering the molecular mechanisms
In addition to insulin, insulin-like growth factor 1 (IGF-1) is also involved in the metabolism of sugar and fat. The effect of this growth factor is weakened by binding to the IGF-binding protein 2 (IGFBP2). If the liver does not release enough IGFBP2 into the blood, the balance of the glucose and lipid metabolism may be disrupted. The research team led by Schürmann and Professor Matthias Schulze, head of the Department of Molecular Epidemiology at DIfE, therefore investigated how the diminished effect of the IGFBP2 gene could influence the development of type 2 diabetes.
Human studies show that people suffering from fatty liver produce and release less IGFBP2. Schürmann's team observed similar effects in earlier mouse experiments, which showed that IGFBP2 levels were already reduced prior to the liver disease. This is due to the transfer of methyl groups at certain sites of the IGFBP2 DNA sequence, which inhibited the gene in the liver. These so-called epigenetic changes are caused, among other things, by lifestyle factors. Such modifications of the DNA in the IGFBP2 gene were also previously detected in blood cells of overweight people with impaired glucose tolerance.
Translational research from mouse to human studies
The interdisciplinary research team led by Schürmann and Schulze used findings from the clinic and laboratory to evaluate blood samples and data from the EPIC Potsdam Study. "This study is a good example of how translational research works: A clinical finding is taken up, analyzed mechanistically in the laboratory and finally examined in a population-wide study," said Schürmann.
Recent analyses by the researchers indicate that inhibition of the IGFBP2 gene promotes type 2 diabetes. In addition, the team of scientists observed that leaner study participants and study participants with lower liver fat levels had higher concentrations of the protective binding protein in the blood. Higher plasma concentrations of IGFBP2 were associated with a lower risk of developing type 2 diabetes in subsequent years. "Our study confirms the hypothesis that the IGF-1 signaling pathway also plays an important role in the development of type 2 diabetes in humans," added Dr. Clemens Wittenbecher, research associate in the Department of Molecular Epidemiology at DIfE and first author of the study.
References
Original Publication
Wittenbecher C, Ouni M, Kuxhaus O, Jähnert, M, Gottmann Pl, Teichmann, A, Meidtner, K, Kriebel, J, Grallert, H, Pischon, T, Boeing, H, Schulze, MB, Schürmann, A. Insulin-like growth factor binding protein 2 (IGFBP-2) and the risk of developing type 2 diabetes. Diabetes 2018 (https://doi.org/10.2337/db18-0620)
Related Articles
Kammel A, Saussenthaler S, Jähnert M, Jonas W, Stirm L, Hoeflich A, Staiger H, Fritsche A, Häring HU, Joost HG, Schürmann A, Schwenk RW. Early hypermethylation of hepatic Igfbp2 results in its reduced expression preceding fatty liver in mice. Human Molecular Genetics 2016 (https://doi.org/10.1093/hmg/ddw121)
Baumeier C, Saussenthaler S, Kammel A, Jähnert M, Schlüter L, Hesse D, Canouil M, Lobbens S, Caiazzo R, Raverdy V, Pattou F, Nilsson E, Pihlajamäki J, Ling C, Froguel P, Schürmann A, Schwenk RW. Hepatic DPP4 DNA Methylation Associates With Fatty Liver. Diabetes 2017 (https://doi.org/10.2337/db15-1716)
Schwenk RW, Jonas W, Ernst SB, Kammel A, Jähnert M, Schürmann A. Diet-dependent alterations of hepatic Scd1 expression are accompanied by differences in promoter methylation. Hormone and Metabolic Research 2013 (https://doi.org/10.1055/s-0033-1348263)
Background Information
Epigenetics
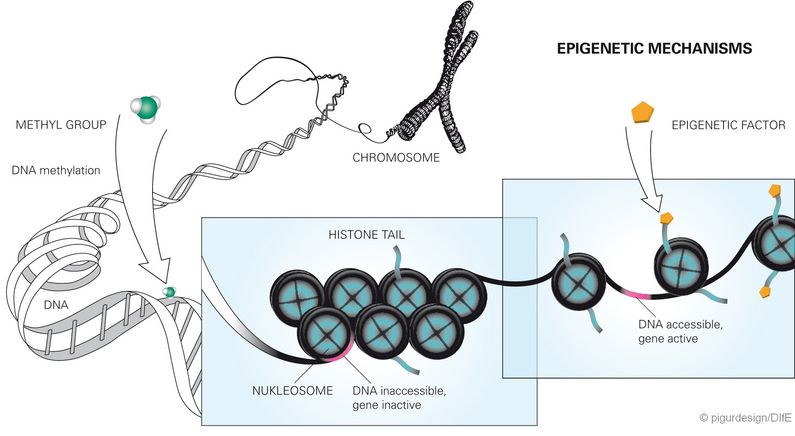
Epigenetics is a relatively young field of research. It investigates altered gene functions that are not attributable to a change in the DNA sequence, but can still be inherited. Recent studies have increasingly suggested that diet as an environmental factor can also have a lasting effect on the activity status of genes, e.g. by chemical (epigenetic) changes of the DNA building blocks. This also includes methylations. These arise when methyl groups bind to the DNA. This can either make the activation of the genes more difficult or easier. The direct methylation of DNA permanently changes gene expression when it occurs in control regions of genes (so-called CpG islands), which have been made accessible by the modification of histones.
EPIC Potsdam Study
The European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study is a prospective cohort study. Between 1994 and 1998, 27,548 women and men between the ages of 35 and 65 were recruited. They completed questionnaires about their eating habits, lifestyle and health status. This survey was repeated approximately every 3 years. The EPIC Potsdam study is part of one of the largest long-term studies worldwide with a total of approximately 521,000 study participants from ten European countries. The aim is to investigate the influence of nutrition on the development of cancer and other chronic diseases.
German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE)
The German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE) is a member of the Leibniz Association. It investigates the causes of diet-related diseases in order to develop new strategies for prevention and therapy and to provide dietary recommendations. Its research focus includes the causes and consequences of the metabolic syndrome, which is a combination of obesity, high blood pressure, insulin resistance and lipid metabolism disorder, as well as the role of diet in healthy aging and the biological basis of food choices and eating habits. In addition, the DIfE is a partner of the German Center for Diabetes Research (DZD), which since 2009 has been funded by the BMBF.
Leibniz Association
The Leibniz Association unites 93 independent research institutions whose focus encompasses the natural, engineering and environmental sciences, economics, the spatial and social sciences as well as the humanities. Leibniz institutes address issues of social, economic and ecological relevance, conduct knowledge-driven and applied basic research, also in the interdisciplinary Leibniz Research Alliances, constitute or maintain scientific infrastructures, and provide research-based services. The Leibniz Association focuses on knowledge transfer, in particular through the Leibniz research museums. It advises and informs policy-makers, academia, business and the public. Leibniz institutions collaborate intensively with universities – for example in the form of “Leibniz ScienceCampi”– as well as with industry and other partners in Germany and abroad. The Leibniz institutions are subject to a transparent and independent evaluation procedure. Due to the importance of the institutions for the country as a whole, the federal government and the states (Länder) jointly fund the institutes of the Leibniz Association, which employs some 19,100 individuals, including 9,900 scientists. The entire budget of all the institutes is approximately 1.9 billion euros.
German Center for Diabetes Research (DZD)
The German Center for Diabetes Research (DZD) is one of six German Centers for Health Research. It brings together experts in the field of diabetes research and integrates basic research, epidemiology, and clinical applications. By adopting an innovative, integrative approach to research, the DZD aims to make a substantial contribution to the successful personalized prevention diagnosis and treatment of diabetes mellitus. The members of the DZD are Helmholtz Zentrum München – German Research Center for Environmental Health, the German Diabetes Center (DDZ) in Düsseldorf, the German Institute of Human Nutrition (DIfE) in Potsdam-Rehbrücke, the Institute of Diabetes Research and Metabolic Diseases of Helmholtz Zentrum München at the University of Tübingen, the Paul Langerhans Institute Dresden of Helmholtz Zentrum München at the Carl Gustav Carus University Hospital of TU Dresden, associated partners at the universities in Heidelberg, Cologne, Leipzig, Lübeck and Munich, and other project partners.
Contact:
Prof. Dr. Annette Schürmann
Department of Experimental Diabetology
German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE)
Arthur-Scheunert-Allee 114-116
14558 Nuthetal/Germany
phone: +49 33200 88-2368
e-mail: schuermann(at)dife.de
Dr. Clemens Wittenbecher
Department of Molecular Epidemiology
German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE)
Arthur-Scheunert-Allee 114-116
14558 Nuthetal/Germany
phone: +49 33200 88-2454
e-mail: Clemens.Wittenbecher(at)dife.de
Media Contact:
Sonja Schäche
Head of Press and Public Relations
German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE)
Arthur-Scheunert-Allee 114-116
14558 Nuthetal/Germany
phone: +49 33200 88-2278
e-mail: sonja.schaeche(at)dife.de
Press contact

Birgit Niesing
niesing(at)dzd-ev.de
+49 (0)89 3187-3971
 |
