Neuherberg, 08.06.2020
Cells Pass on Metabolic Transcriptional Memory to their Progeny
The capacity to remember is essential for all organisms. “Transcriptional memory” enables cells to adapt to changing environments such as food availability. But how do cells pass on memories to their progeny? A team of researchers, with Dr. Poonam Bheda and Prof. Robert Schneider from the Institute of Functional Epigenetics (IFE) at the Helmholtz Zentrum München, focused on this question and published their recent findings in Molecular Cell.
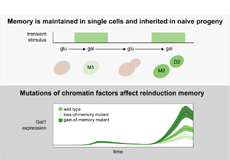
In eukaryotes DNA is packaged into a structure called chromatin. Through epigenetic mechanisms, parts of chromatin can be opened or closed and thereby become – or not – accessible for the transcription machinery. Epigenetic mechanisms thus function like switches that can respond to environmental stimuli. The capacity to remember the transcriptional response to a certain stimulus enables cells to react faster if they encounter the same stimulus again. This so called “transcriptional reinduction memory” is advantageous for cell survival. In humans it is implicated in environmentally triggered diseases, such as diabetes. Thus, unravelling how transcriptional memory is regulated and how it is passed on from a cell to its progeny is key to understanding the transmission of disease states.
Researchers from the IFE developed and applied a novel workflow combining microfluidics with single-cell live imaging to construct pedigrees and analyze transcriptional changes and memory in single cells over multiple generations. “Microfluidics traps individual cells, which makes it possible to monitor their behavior and importantly to trace their ancestry”, said Dr. Poonam Bheda.
The researchers studied the galactokinase GAL1* gene in yeast as a model for nutrient driven transcriptional memory. To determine how inheritance of transcriptional memory is regulated the team performed a high-throughput screen to identify factors controlling this memory. “We focused on chromatin factors because we hypothesized that epigenetic mechanisms would regulate memory of metabolic states, and this is what we found”, said Prof. Robert Schneider. Altogether, this work provides an important new tool to study transcriptional memory by dissecting memory maintenance and inheritance.
In humans, transcriptional memory might be a driver of diabetes progression. The reaction of cells to high blood sugar** has tremendous effects on the patients as cells can become resistant to insulin. This approach could thus be used to uncover the molecular mechanisms behind insulin resistance for example, and contribute to the development of new diabetes therapies.
Original publication:
Bheda et al. (2020). Single-cell tracing dissects regulation of maintenance and inheritance of transcriptional reinduction memory. Molecular Cell. DOI: 10.1016/j.molcel.2020.04.016
Further information:
For background information to metabolic transcriptional memory, please check out this review article: Poonam Bheda (2020). Metabolic transcriptional memory. Molecular Metabolism. DOI: 10.1016/j.molmet.2020.01.019.
* In yeast, galactose is the non-preferred carbon source for growth. However, in case glucose is not available, yeast metabolizes galactose to extract energy. Therefore, the cells express different enzymes that break down galactose including the galactokinase GAL1. Cells that have never been in contact with galactose need some time until the galactose-metabolism machinery starts, whereas cells that had to switch on the machinery before can react faster when they are exposed to galactose again.
** In human, high blood sugar levels (hyperglycemia), even if only for a short period, can cause metabolic memory that leads to long-lasting complications. These effects are largely irreversible, even if the sugar levels are under control, and accumulate to drive obesity, diabetes, and ageing.
Press contact

Birgit Niesing
niesing(at)dzd-ev.de
+49 (0)89 3187-3971